Monday, November 30, 2015
Brilliant Time-Lapse of Alaska’s Northern Lights
The beautiful interplay of charged particles from outer space (electrons and protons) with single atoms of oxygen! If you're interested, read more at this Wikipedia link.
Topics for Unit 6 Bonding Test
Bonding Unit Topics (Test Dec 4)
General Bonding:
Octet Rule
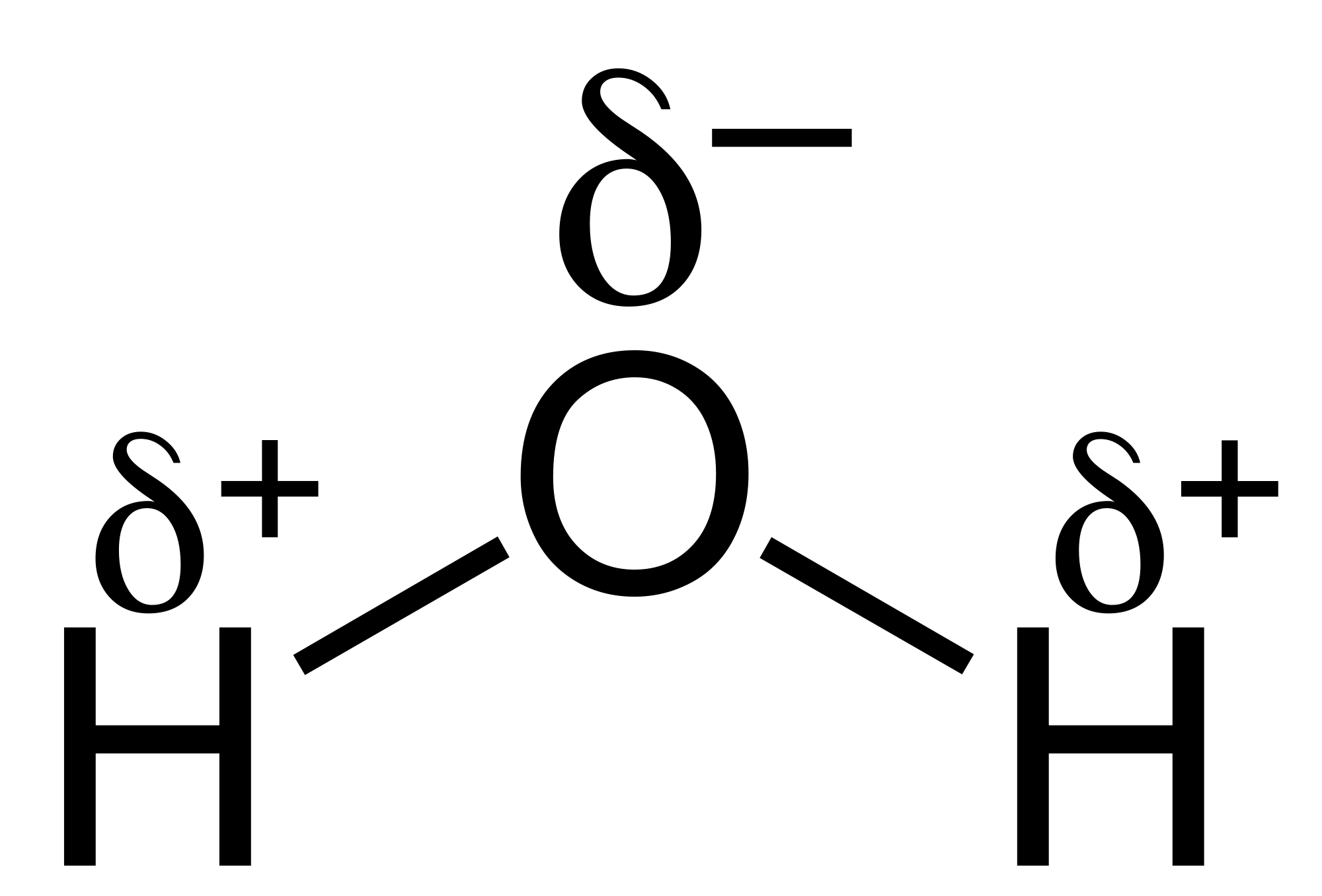
Electronegativity and Bond Type (non polar bonds, polar bonds, ionic bonds)
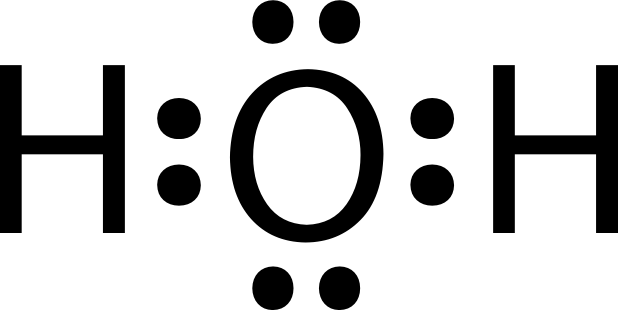
Lewis Dot Diagrams
Valence electrons for each family (Main Group columns: 1A, 2A, 3A, 4A, 5A, 6A, 7A, 8A)
Electronegativity and Bond Type (non polar bonds, polar bonds, ionic bonds)
Lewis Dot Diagrams
Valence electrons for each family (Main Group columns: 1A, 2A, 3A, 4A, 5A, 6A, 7A, 8A)
Covalent Bonds
General properties (gases, liquids or soft solids at room temp)
Lewis Structures
Single, double and triple bonds (Hybridization: sp3, sp2, sp)
VSEPR shapes (linear, bent, trigonal planar, trigonal pyramidal, tetrahedral)
Polar vs nonpolar molecules
Ionic Bonds
Ionic Crystals
Properties of ionic compounds as described by ionic bonds
Brittle Solids at room temperature
High melting and boiling points
Don’t conduct electricity in solid state
Conduct electricity when molten or dissolved in a polar liquid.
Metallic Bonds
Mobile sea of valence electrons attracted to metal cation
Properties of metals as explained by metallic bonding.
luster, malleable, ductile, good conductors of heat and electricity
Alloys
Intermolecular Forces (IMF)
London Dispersion Forces
Dipole - Dipole Forces
Hydrogen Bonding
Tuesday, November 24, 2015
Homework due 11.30.15
Read
Chapter 14.1A, pp 489-491
Do
Problems 1, 3, 5, 7 pg 514
note: Practice test for Unit 6 is in Chemistry Locker
Chapter 14.1A, pp 489-491
Do
Problems 1, 3, 5, 7 pg 514
note: Practice test for Unit 6 is in Chemistry Locker
Monday, November 23, 2015
Homework due 11.24.15
Read
Chapter 12.4C, pp 430-433
Do
Problems 50 (b,c), 51, 52 (hint: read the PDF I posted earlier for a direct answer to this) pg 437
Chapter 12.4C, pp 430-433
Do
Problems 50 (b,c), 51, 52 (hint: read the PDF I posted earlier for a direct answer to this) pg 437
Saturday, November 21, 2015
Friday, November 20, 2015
Thursday, November 19, 2015
Wednesday, November 18, 2015
Tuesday, November 17, 2015
Homeword due 11.18,19.15
Read
Chapter 12.3A, pp 413-416
Do
Practice Problem • Exercise 12.2 pg 417 (top of page)
Problems 30, 33, 34 pg 436
Chapter 12.3A, pp 413-416
Do
Practice Problem • Exercise 12.2 pg 417 (top of page)
Problems 30, 33, 34 pg 436
Monday, November 16, 2015
Thursday, November 12, 2015
Wednesday, November 11, 2015
Agenda for 11.12.15
Topics for Friday Test
- Ionic Compounds
- Binary with main group elements
- Binary with Transition Metals
- Polyatomic Ions
- (chart with ions provided)
- Molecular Compounds
- Prefixes and when to use them
- Diatomic 7 (Horses Need Oats For Clear Brown I’s?)
- molecular compounds with common names (H2O, NH3, H2O2)
- Acids
- monoatomic anion
- polyatomic anions
You must be able to go back and forth between name and formula!
Friday, November 6, 2015
Homework due 11.9.15
Read
Chapter 4.2 pp 109-117
Do
Problems 17, 18, 20, 22, 23, 26, 27 pg 119
click here fore awesome ionic naming game!
Chapter 4.2 pp 109-117
Do
Problems 17, 18, 20, 22, 23, 26, 27 pg 119
click here fore awesome ionic naming game!
Monday, November 2, 2015
Homework due 11.4,5.15
Read
Chapter 4.1B, pp 113-114
Do
questions on p 118, #10, 11
Chapter 4.1B, pp 113-114
Do
questions on p 118, #10, 11
Homework due 11.3.15
Read
Chapter 4.1, section A, pp 92-103
Do
Problems 4, 5, 6, 7, pg 118
Chapter 4.1, section A, pp 92-103
Do
Problems 4, 5, 6, 7, pg 118
Subscribe to:
Comments (Atom)