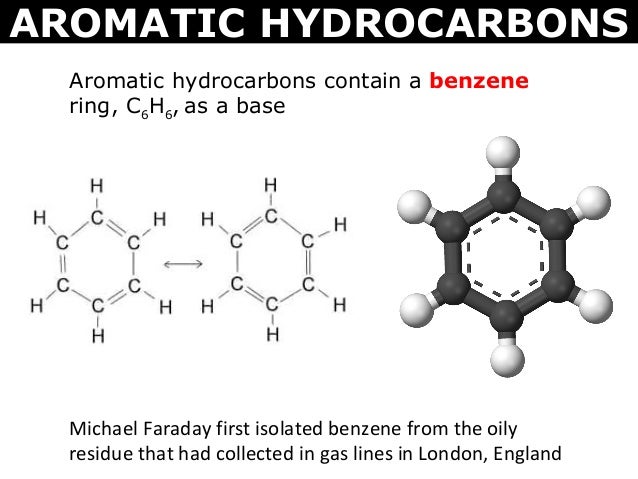
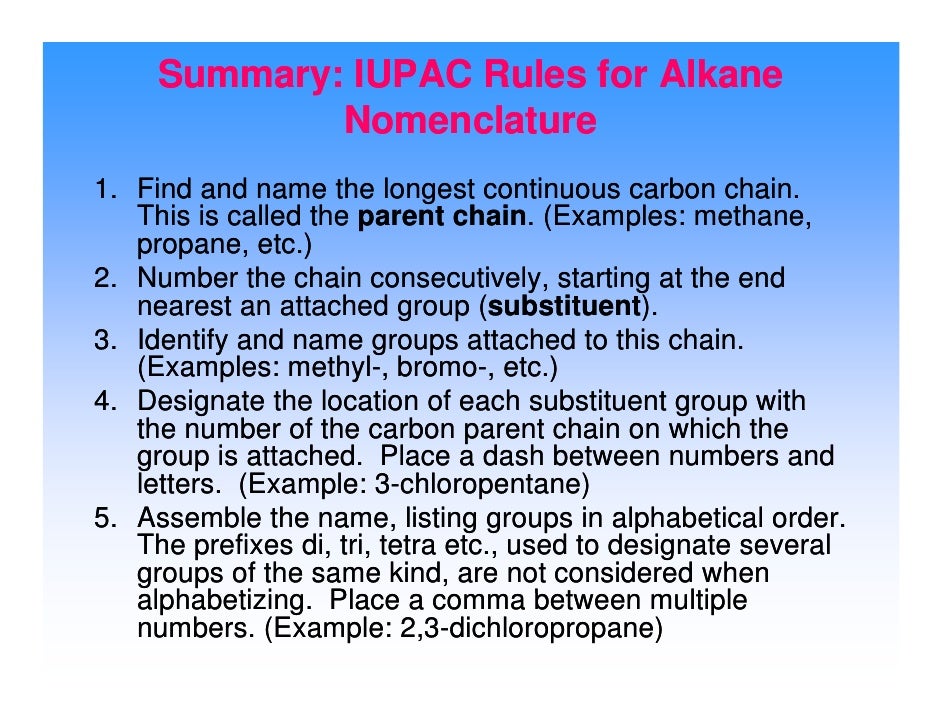
in case you lose your piece of paper:
Here it is!
Remember, some students have a version with the error in the last problem. Be careful. Your version has been corrected.
Wednesday, December 13, 2017
Tuesday, December 12, 2017
Monday, December 11, 2017
Wednesday, December 6, 2017
Friday, December 1, 2017
Wednesday, November 29, 2017
Tuesday, November 28, 2017
Wednesday, November 15, 2017
Homework and Agenda for Thursday 11.16.17
Read
Chapter 12.10 pp 390-392
Supplemental Handout on hybrid molecular orbitals
Do
Problems 48bc, 49bc, 50bd, 51, 52
Thursday
Quiz on major Chapter 11, 12, and general classroom topics
Vocabulary
Chapter 12.10 pp 390-392
Supplemental Handout on hybrid molecular orbitals
Do
Problems 48bc, 49bc, 50bd, 51, 52
Thursday
Quiz on major Chapter 11, 12, and general classroom topics
Vocabulary
- Fluorescence
- Fungible
- Isoelectronic
- Orbital
- Energy Level
- s, p, d, f
- Key Terms page 354, page 394
Tuesday, November 14, 2017
Monday, November 13, 2017
Thursday, November 9, 2017
Homework due Monday 11.13.17
Read
Chapter 12.5, 12.6 pp 369-375
Do
Problems 24, 26, 28bd, 29bd, 30, 33bd, 34bd, pp 395-396
Chapter 12.5, 12.6 pp 369-375
Do
Problems 24, 26, 28bd, 29bd, 30, 33bd, 34bd, pp 395-396
Wednesday, November 8, 2017
Tuesday, November 7, 2017
Homework for Wednesday 11.8.17
[note: start Chapter 12 HW on a new sheet of paper!]
Read
Chapter 12.1, 12.2, pp 359-363
Do
Problems 1, 2, 4, 9, 10, 11, 12
Read
Chapter 12.1, 12.2, pp 359-363
Do
Problems 1, 2, 4, 9, 10, 11, 12
Monday, November 6, 2017
Homework/Agenda for Tuesday, 11.7.17
Homework Harvest for Chapters 6, 9
Read
You've already read the whole chapter!
Do
Critical Thinking Problems 62, 66, 68, 69, 70, 71, pg 357
Read
You've already read the whole chapter!
Do
Critical Thinking Problems 62, 66, 68, 69, 70, 71, pg 357
Friday, November 3, 2017
Homework and Agenda for Monday 11.6.17
Read
Chapter 11.11, pp 348-352
Do
Problems 49, 50, 53, black only on 55, 56, 57
Flame Test Lab on Monday
Emission Lines of the Elements
Chapter 11.11, pp 348-352
Do
Problems 49, 50, 53, black only on 55, 56, 57
Flame Test Lab on Monday
Emission Lines of the Elements
Thursday, November 2, 2017
Friday, October 27, 2017
Thursday, October 26, 2017
Homework for Friday 10.27.17
Read
Chapter 11.3, 11.4, pp 326-330
Do
Problems 10, 11, 12, 13, 14, 15 pg 355
Chapter 11.3, 11.4, pp 326-330
Do
Problems 10, 11, 12, 13, 14, 15 pg 355
Wednesday, October 25, 2017
Thursday, October 19, 2017
Agenda for Wednesday, 10.25.17
Chapter 6, 9 Test
Also be prepared to answer any questions about the demo reactions we did:
Also be prepared to answer any questions about the demo reactions we did:
- Sodium in water
- Whoosh Bottle
- Burning of Mg in air
Agenda and Homework for Tuesday 10.24.17
Dry Lab Quiz
Homework
Chapter 8 HW Harvest!
- Determining the formula of a hydrate
- Moles of Chalk in your name
Homework
Chapter 8 HW Harvest!
- Read
- Chapter 9.7, 9.8, pp 271-279
- Do
- Problems 35b,d, 36b,d, 37, 45
Homework due 10.20.17
Read
Chapter 9.3, 9.4, pp 258-265
Do
Problems 13, 14, 15 (black only for these), 27
Chapter 9.3, 9.4, pp 258-265
Do
Problems 13, 14, 15 (black only for these), 27
Tuesday, October 17, 2017
Homework due 10.19.17
Read
Chapter 9.1, 9.2, pp 251-258
Do
Problems 3, 4, 8, 9, 10 (black only) pp 281-282
Chapter 9.1, 9.2, pp 251-258
Do
Problems 3, 4, 8, 9, 10 (black only) pp 281-282
Friday, October 13, 2017
Homework due 10.16.17
Read
Chapter 6.5, 6.6, pp 171-176
Do
Problems (black only) 27, 30, 31, 33, 34 pg 189
Chapter 6.5, 6.6, pp 171-176
Do
Problems (black only) 27, 30, 31, 33, 34 pg 189
Tuesday, October 10, 2017
Thursday, October 5, 2017
Homework due Friday, 10.6.17
Tuesday, October 3, 2017
Homework due Wednesday 10.4.17
Read
You already read the whole chapter!
Do
Problems 37, 38, 39 (black problems only)
You already read the whole chapter!
Do
Problems 37, 38, 39 (black problems only)
Monday, October 2, 2017
Friday, September 29, 2017
Thursday, September 28, 2017
Homework due Friday, 9.29.17
Read
Chapter 8.3, 8.4, pp 223-230
Do
Problems 14 (black), 16, 17, 22, 23 (black only for 22, 23)
Chapter 8.3, 8.4, pp 223-230
Do
Problems 14 (black), 16, 17, 22, 23 (black only for 22, 23)
Wednesday, September 27, 2017
Homework due Thursday 9.28.17
[reminder: Ch 4, 7 HW Harvest tomorrow!]
Read
Chapter 8.1, 8.2, pp 212-223
Do
Problems 1, 2, 5, 6, 8, 9 pp 245-246
[note: only do 'black' problems for #8 and #9]
Read
Chapter 8.1, 8.2, pp 212-223
Do
Problems 1, 2, 5, 6, 8, 9 pp 245-246
[note: only do 'black' problems for #8 and #9]
Tuesday, September 26, 2017
Homework due Wednesday, 9.27.17
Do
Chapter 7 Critical Thinking Problems: 38, 42, 45, 46, 49, pg 211
Chapter 4, 7 HW Harvest on Thursday, 9/28/17
Chapter 7 Critical Thinking Problems: 38, 42, 45, 46, 49, pg 211
Chapter 4, 7 HW Harvest on Thursday, 9/28/17
Monday, September 25, 2017
Homework due Tuesday 9.26.17
Read
Chapter 7.3, pp 200-207
Do
Question 31, pg 210
Problems 33, 34, pg 210 (just do 'black' problems b, d)
Chapter 7.3, pp 200-207
Do
Question 31, pg 210
Problems 33, 34, pg 210 (just do 'black' problems b, d)
Saturday, September 23, 2017
Friday, September 22, 2017
Thursday, September 21, 2017
Periodic Table Test on Monday!
You need to know the first 36 elements:
- name
- atomic number
- location on periodic table
You also need to know which columns are the following groups/families:
- Alkalai Metals
- Alkaline Earth Metals
- Halogens
- Noble Gases
And oh, BTW, here's a cool link to some periodic tables:
Wednesday, September 20, 2017
Homework due Thursday 9.21.17
Read
Chapter 4.6, pp 105-106
Do
Problems (only do the black problems) 25, 26, 27 pg 109
Chapter 4.6, pp 105-106
Do
Problems (only do the black problems) 25, 26, 27 pg 109
Tuesday, September 19, 2017
Homework due Wednesday 9.20.17
Read
Chapter 4.3, 4.4, pp 97-104
Do
Problems 15, 17, 18, 21 pp 108-109
Big 4 Polyatomic Ions mini-quiz on Wednesday!
Periodic Table Quiz on Monday!
Chapter 4.3, 4.4, pp 97-104
Do
Problems 15, 17, 18, 21 pp 108-109
Big 4 Polyatomic Ions mini-quiz on Wednesday!
Periodic Table Quiz on Monday!
Monday, September 18, 2017
Friday, September 15, 2017
Homework due Monday 9.18.17
Homework Harvest on Monday!
Chapters 3, 5
Read
Chapter 4.1 pp 85-94
Do
Questions 3,6,7,8 pg 108
Wednesday, September 13, 2017
Homework due 9.14.17
Read
Chapter 5.6, 5.7, 5.8 pp 130-147
Do
Problems 35, 36, 41, 43, 48, 58, 61 pp 150-151
Chapter 5.6, 5.7, 5.8 pp 130-147
Do
Problems 35, 36, 41, 43, 48, 58, 61 pp 150-151
Tuesday, September 12, 2017
Homework due 9.13.17
Read
Chapter 5.3, 5.4, 5.5 pp 118-129
Do
Problems 15, 17, 19, 20, 22, 24, 25 pg 149
Chapter 5.3, 5.4, 5.5 pp 118-129
Do
Problems 15, 17, 19, 20, 22, 24, 25 pg 149
Monday, September 11, 2017
Friday, September 8, 2017
Thursday, September 7, 2017
Wednesday, September 6, 2017
Homework for Thursday 9.7.17
Read
Chapter 3.7, 3.8 pp 60 - 67
Do
Problems 23, 25, 27, 28, 29, 30, 35, 37, 39 pg 82
Chapter 3.7, 3.8 pp 60 - 67
Do
Problems 23, 25, 27, 28, 29, 30, 35, 37, 39 pg 82
Tuesday, September 5, 2017
Friday, September 1, 2017
Homework for Tuesday, 9.5.17
Read
Chapter 3.1, 3.2, 3.3, pp 47-53
Do
Problems 3, 5, 7, 10, 12
Note: Homework Harvest on Tuesday for Chapters 1 and 2. Don't forget to bring all your stamped homework!
Chapter 3.1, 3.2, 3.3, pp 47-53
Do
Problems 3, 5, 7, 10, 12
Note: Homework Harvest on Tuesday for Chapters 1 and 2. Don't forget to bring all your stamped homework!
Wednesday, August 30, 2017
Tuesday, August 29, 2017
Homework due Wednesday 8.30.17
Read
Chapter 2.3, 2.4, 2.5 pp 28-38
Do
Problems 5, 7, 8, 12, 13, 14, 15
Chapter 2.3, 2.4, 2.5 pp 28-38
Do
Problems 5, 7, 8, 12, 13, 14, 15
Monday, August 28, 2017
Thursday, August 24, 2017
Online version of the textbook
In order to access this link, you must be signed into your SFUSD Google account:
https://drive.google.com/open?id=0BzppquxFcqpRSDN5SC1MZ044Sms
https://drive.google.com/open?id=0BzppquxFcqpRSDN5SC1MZ044Sms
Wednesday, August 23, 2017
Thursday, May 4, 2017
Monday, May 1, 2017
Homework for 5.2.17
Read
Chapter 20.10, 20.11, 20.12, pp 660 - 664
Do
Problems 38, 39, 42, 43, 47
Chapter 18 HW Harvest tomorrow (Tuesday)
Here are the functional groups you need to know (Those without the big X)
Chapter 20.10, 20.11, 20.12, pp 660 - 664
Do
Problems 38, 39, 42, 43, 47
Chapter 18 HW Harvest tomorrow (Tuesday)
Here are the functional groups you need to know (Those without the big X)
Tuesday, April 25, 2017
Monday, April 24, 2017
Friday, April 21, 2017
Homework due 4.24.17
Tuesday, April 18, 2017
Building your cheat sheet for Friday
Look at this sheet:
http://chemistry.ggould.com/Study_Guides/StandardElectrodePotentials.html
Please leave your comments below to add items to the "Cheat Sheet"
http://chemistry.ggould.com/Study_Guides/StandardElectrodePotentials.html
Please leave your comments below to add items to the "Cheat Sheet"
Monday, April 17, 2017
Homework and Agenda due 4.21.17
Read
Chapter 18.8, pp 599 - 600
Do
Problems 39, 42, 49, 51
Chapter 18.8, pp 599 - 600
Do
Problems 39, 42, 49, 51
Chapter 18/electrochem assessment on Friday 4.21.17
Eocell = Eoreduction + Eooxidation
Friday, April 14, 2017
Monday, April 10, 2017
Homework due Friday 4.14.17
Read
Chapter 18.5, pp 591 - 594
Do
Problems 28, 30 pg 604
There will be a quiz Friday on 3 demos I hope to do this week:
Chapter 18.5, pp 591 - 594
Do
Problems 28, 30 pg 604
There will be a quiz Friday on 3 demos I hope to do this week:
- Thermite
- Elephant's Toothpaste
- Electrolysis of Water (student lab if we have enough supplies)
Thursday, April 6, 2017
Tuesday, April 4, 2017
Monday, April 3, 2017
Homework/Agenda for 4.4.17
Read
Chapter 18.1, 18.2, pp 574 - 579
Do
Problems 3b,c, 4b,d, 9b,d, 11b,d, 13b,d
Chapter 16-17 HW Harvest on Wednesday
Chapter 18.1, 18.2, pp 574 - 579
Do
Problems 3b,c, 4b,d, 9b,d, 11b,d, 13b,d
Chapter 16-17 HW Harvest on Wednesday
Friday, March 24, 2017
Wednesday, March 22, 2017
Homework Due 3.23.17
Read
Chapter 17.9, 17.10, pp 562 - 567
Do
Problems 36, 39, 46, 48, 53, pp 572 - 573
Chapter 17.9, 17.10, pp 562 - 567
Do
Problems 36, 39, 46, 48, 53, pp 572 - 573
Tuesday, March 21, 2017
Homework due 3.22.17
Monday, March 20, 2017
Homework Due 3.21.17
[note: "Common Sense" Equilibrium Quiz on Thursday. ]
Read
Chapter 17.7, pp 552 - 554
Do
Problems 25, 26, pg 571
- No Notes
- No Calculators
- No Kidding!
Read
Chapter 17.7, pp 552 - 554
Do
Problems 25, 26, pg 571
Friday, March 17, 2017
Thursday, March 16, 2017
Tuesday, March 14, 2017
Wednesday, March 8, 2017
Tuesday, March 7, 2017
Monday, March 6, 2017
Strong Acids
These are the strong acids you should know about. All others are 'weak.'Reducing Agents)
- HCl hydrochloric acid
- HBr hydrobromic acid
- HI hydroiodic acid
- HClO4 perchloric acid
- HNO3 nitric acid
- HIO4 periodic acid
- H2SO4 sulfuric acid
- HClO3 chloric acid
Wednesday, March 1, 2017
Homework due 3.6.17
Read
Chapter 16.4 pp 513 - 520
Do
Problems 28, 29, 30, 31b, 31d, 32b, 32d, 34b, 35b, 36b
Warning: This homework is not due until Monday. Do not wait until the last minute to start looking at this. You need some time to get used to these kinds of problems.
Click here to download/view Study Guide for Chapter 16
Chapter 16.4 pp 513 - 520
Do
Problems 28, 29, 30, 31b, 31d, 32b, 32d, 34b, 35b, 36b
Warning: This homework is not due until Monday. Do not wait until the last minute to start looking at this. You need some time to get used to these kinds of problems.
 |
| The ideal pH for discus is around 6.5 |
Tuesday, February 28, 2017
Monday, February 27, 2017
Homework due 2.28.17
Read
Chapter 16.2, pp 506 - 510
Do
Problems 13, 15, 16
Lab prep for Tuesday:
Please read instructions for this lab, as time is limited, and we won't have enough time if you don't prepare.
Chapter 16.2, pp 506 - 510
Do
Problems 13, 15, 16
Lab prep for Tuesday:
Please read instructions for this lab, as time is limited, and we won't have enough time if you don't prepare.
pH Lab
Test-tubes with a capacity of around 10 mL are ideal. The test-tubes should be as clean as possible. Test-tubes, dropping pipettes and measuring cylinders should be washed in tap water and then rinsed with deionised or distilled water.
Procedure
Students 1 and 2
Test-tubes with a capacity of around 10 mL are ideal. The test-tubes should be as clean as possible. Test-tubes, dropping pipettes and measuring cylinders should be washed in tap water and then rinsed with deionised or distilled water.
Procedure
Students 1 and 2
a Number the test-tubes 1–7.
b Half-fill test-tube 1 with the hydrochloric acid solution.
c Transfer 1 mL of the hydrochloric acid into the measuring cylinder. Add distilled or deionised water to the measuring cylinder, up to the 10 mL mark.
d Pour some of the resulting diluted solution from the measuring cylinder into test-tube 2, enough to come to a similar height as the solution in test-tube 1.
e Carefully, pour away all but 1 mL of the solution remaining in the measuring cylinder. Now add distilled or deionised water to the measuring cylinder up to the 10 mL mark. Pour the resulting solution into test-tube 3. Continue in this way until you have solutions in test-tubes 1 to 6. Put only distilled or deionised water into test-tube 7.
Students 3 and 4
f Repeat instructions a – e using the sodium hydroxide solution instead of hydrochloric acid. Number the test-tubes 8–13.
Both groups
g Put the two racks of test-tubes together so that the solutions are in order 1 to 13. The test-tubes now have solutions in them with pH 1 (test-tube 1) to pH 13 (test-tube 13).
h Add a drop of Universal indicator to each test-tube. Rock each test-tube from side to side to mix the contents. Add more Universal indicator solution to each test-tube if needed to allow the colours to be seen more clearly. Be sure to add the same number of drops of indicator to each test-tube.
i Compare the colors of the solutions with the pH indicator chart.
b Half-fill test-tube 1 with the hydrochloric acid solution.
c Transfer 1 mL of the hydrochloric acid into the measuring cylinder. Add distilled or deionised water to the measuring cylinder, up to the 10 mL mark.
d Pour some of the resulting diluted solution from the measuring cylinder into test-tube 2, enough to come to a similar height as the solution in test-tube 1.
e Carefully, pour away all but 1 mL of the solution remaining in the measuring cylinder. Now add distilled or deionised water to the measuring cylinder up to the 10 mL mark. Pour the resulting solution into test-tube 3. Continue in this way until you have solutions in test-tubes 1 to 6. Put only distilled or deionised water into test-tube 7.
Students 3 and 4
f Repeat instructions a – e using the sodium hydroxide solution instead of hydrochloric acid. Number the test-tubes 8–13.
Both groups
g Put the two racks of test-tubes together so that the solutions are in order 1 to 13. The test-tubes now have solutions in them with pH 1 (test-tube 1) to pH 13 (test-tube 13).
h Add a drop of Universal indicator to each test-tube. Rock each test-tube from side to side to mix the contents. Add more Universal indicator solution to each test-tube if needed to allow the colours to be seen more clearly. Be sure to add the same number of drops of indicator to each test-tube.
i Compare the colors of the solutions with the pH indicator chart.
Tuesday, February 21, 2017
Homework/Agenda for 2.22.17
Solutions (Chapter 15) test on Friday
Ice Cream making on Thursday
Bring (everybody)
Read
Chapter 16.1, pp 503 - 506
Do
Problems 7 b,d, 8 b,c, 9 b,d, 10 b,d
Ice Cream making on Thursday
Bring (everybody)
- 1 qt Ziplok bag
- 1 gal Ziplok bag
- spoons
Read
Chapter 16.1, pp 503 - 506
Do
Problems 7 b,d, 8 b,c, 9 b,d, 10 b,d
Friday, February 17, 2017
Homework due 2.21.17
Read
Chapter 15.10, pp 495 - 496
Do
Problems 50, 51, 59
Calculation of Freezing Point Depression
Chapter 15.10, pp 495 - 496
Do
Problems 50, 51, 59
Calculation of Freezing Point Depression
If the solution is treated as an ideal solution, the extent of freezing-point depression depends only on the solute concentration that can be estimated by a simple linear relationship with the cryoscopic constant ("Blagden's Law"):
- ΔTF = KF · b · i,
where:
- ΔTF, the freezing-point depression, is defined as TF (pure solvent) − TF (solution).
- KF, the cryoscopic constant, which is dependent on the properties of the solvent, not the solute. (Note: When conducting experiments, a higher KF value makes it easier to observe larger drops in the freezing point. For water, KF = 1.853 K·kg/mol.[7])
- b is the molality (moles solute per kilogram of solvent)
- i is the van 't Hoff factor (number of ion particles per individual molecule of solute, e.g. i = 2 for NaCl, 3 for BaCl2)
Thursday, February 16, 2017
Homework due 2.17.17
Homework
|
Read Chapter 15.8, 15.9, pp 488 - 495
Do Problems 39, 41b,d, 44b,c, 48, pg 501 |
Tuesday, February 14, 2017
Subscribe to:
Comments (Atom)
.svg/350px-Decay_chain(4n%2B2%2C_Uranium_series).svg.png)